ALT-100
What is the protein eNAMPT?
Aqualung Scientists advanced a functional genomic research program designed to identify novel candidate genes involved in contributing to the staggering mortality of inflammatory disorders such as ARDS. We discovered nicotinamide phosphoribosyltransferase (NAMPT) as a novel therapeutic & biomarker for conditions such as Acute Respiratory Distress Syndrome and Sepsis. We demonstrated that extracellular NAMPT is a cytokine triggering the powerful NFkB-dependent inflammatory signaling cascade. What did this early research tell us? NAMPT is detectable in the blood and when triggered due to some external stimuli we know the following:

It is an ideal Target
Expression is induced by trauma, infection, ventilator use, and radiation

Biomarker
NAMPT plasma levels are increased in ARDS, trauma, radiation, cancer

Risk Factor
NAMPT genetic variants are linked to ARDS risk & mortality, cancer
We demonstrated that NAMPT works to amplify unchecked inflammation by binding a key and powerful inflammatory receptor called Toll-like Receptor 4 (TLR4). We have now developed a monoclonal antibody ALT-100 to inhibit eNAMPT binding to TLR4. ALT-100 is a novel and potential game-changing therapy for many inflammatory conditions such as Acute Respiratory Distress Syndrome, Ventilator Induced Lung Injury, Radiation Induced Lung Injury, Chorioamnionitis, Prostate Cancer, Pulmonary Hypertension and Pulmonary Fibrosis.
We further demonstrated in preclinical models that NAMPT neutralizing antibodies or inhibitors attenuated various inflammatory lung disorders such as Acute Respiratory Distress Syndrome, Sepsis, Ventilator Induced Lung Injury and pulmonary hypertension. It was also proven that NAMPT binds directly Toll-like Receptor 4 (TLR4) and exhibits a sequence homologous to MD-2, a known TLR4 cofactor binding protein. NAMPT via TLR4 binding, activates NFkB and innate immunity responses independent of any co-factors. We have now developed a monoclonal antibody ALT-100 to inhibit against this ligand receptor binding region and treat many inflammatory conditions such as Acute Respiratory Distress Syndrome, Ventilator Induced Lung Injury, Radiation Induced Lung Injury, Chorioamnionitis, Prostate Cancer, Pulmonary Hypertension and Pulmonary Fibrosis.
How to target eNAMPT?
With a novel therapeutic drug called ALT-100
Aqualung Therapeutics Corporation has developed a “First in Man” immune-based monoclonal antibody (mAb), known as ALT-100. ALT-100 is designed to address and prevent the development of “unchecked inflammation” in a number of severe inflammatory diseases.
Aqualung Therapeutics has worked collaboratively with Fusion Antibodies to develop a superior therapeutic antibody. Aqualung’s ALT-100 antibody is a revolutionary, next generation antibody therapy. The humanized antibody has been developed and optimized by Fusion Antibodies’ revolutionary CDRx humanization and ADD developability platforms. This humanized monoclonal antibody will block systemic inflammation and will improve survival in the following disease states:
Aqualung Therapeutic’s lead indication for the drug ALT-100 is for the treatment and prevention of Acute Respiratory Distress Syndrome(ARDS) & Ventilator Induced Lung Injury(VILI).
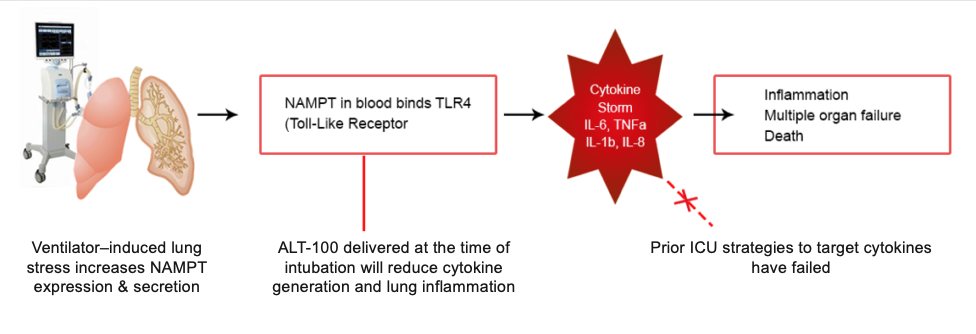
Delivered intravenously, ALT-100 neutralizes circulating eNAMPT, thus reducing the potential for development of unchecked inflammation that affect the mortality of diverse inflammatory diseases such as Acute Respiratory Distress Syndrome, Ventilator-Induced Lung Injury, and Trauma-Induced Lung Lung Injury. By greatly attenuating NAMPT-mediated inflammation, delivery of ALT-100 will improve survival in the critically ill and in patients with respiratory failure as well as patients at risk for VILI following extensive and complicated surgeries. ALT-100 is an innovative mAb therapy, that is designed to be prophylactically-administered at the time of patient intubation for respiratory failure and initiation of Mechanical Ventilation (i.e. before VILI develops). Importantly, because of advances in Precision Medicine strategies by the Aqualung Team, based on blood levels of eNAMPT and NAMPT genetic variants, we can select individuals who are most likely to benefit from ALT-100.
For subacute and chronic disorders, ALT-100 can be delivered at the time of initiated radiotherapy, or at the time of transition from indolent to metastatic prostate cancer. This approach distinguishes ALT-100 therapy from the many failed drugs that targeted a single cytokine (i.e. TNF-a, IL-1b, IL-6 etc.) which were delivered after the presence of established lung injury and inflammation. Because levels of multiple cytokines are already markedly elevated, a process known as “cytokine storm”, these single cytokine-directed therapies were ineffective in reducing inflammation. In the absence of newer therapies focused exclusively on unchecked inflammation, and with unacceptable ICU mortality rates ALT-100 mAb therapy will create a new market and is expected to improve outcomes, reduce healthcare costs, save lives and provide cost savings to hospitals and insurers.